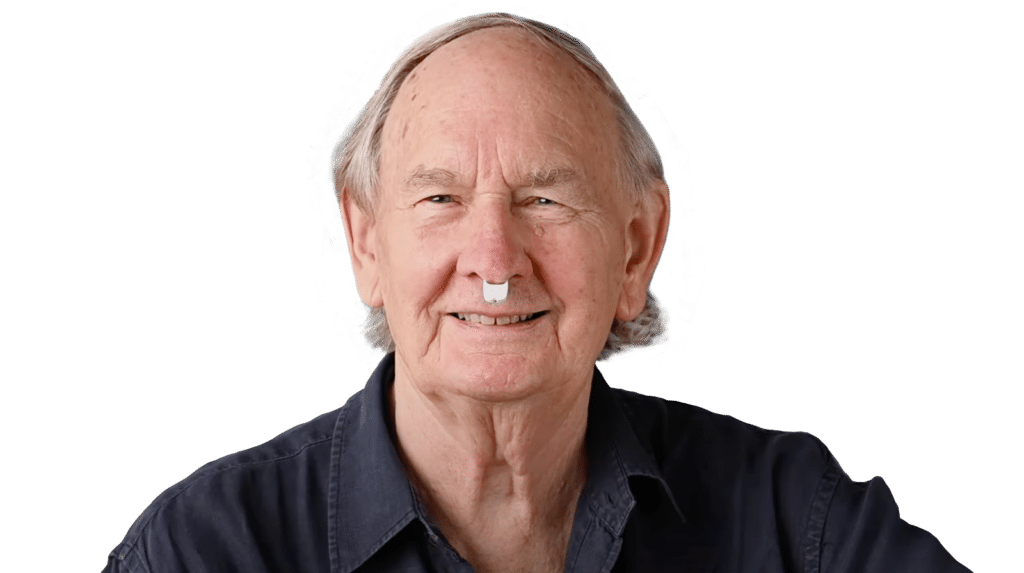
Introducing rhinoswab
Designed for Reliability and Comfort
Clinically equivalent to combined nose and throat swab
Standardized, consistent sampling process
Comfortable and pain free
Preferred by users over standard swabs
Easy to use
Rhinoswab is listed for sale as a Class 1 device in Europe (CE Mark), in the United States with the US Food and Drug Administration (FDA), in the UK with the Medicines and Healthcare products Regulatory Agency (MHRA), in Canada with a Medical Device Establishment Licence (MDEL) and in Australia with the Therapeutic Goods Administration (TGA).
A new standard in sample collection
Standardized, REliable sample self-collection to optimize accuracy
Rhinoswab’s standardized sample collection process provides a 1.4 times larger sample for testing than the regular Copan ESwab to optimize the reliability of the test result.
The full results of the Rhinoswab Sample Capture Study can be found here.

Rhinoswab’s standardized sample collection process is pain free and easy to follow.
Rhinoswab is compatible with both laboratory based RT-PCR testing and rapid antigen testing.
Clinical Validation
Rhinoswab comparable to combined throat and nose swab in detecting covid
Evaluation of a novel anterior nasal swab for the detection of SARS-CoV-2
Amy Crowe, Yves Poy Lorenzo, Darren Jardine, Kumar Visvanathan, Evaluation of a novel anterior nasal swab for the detection of SARS-CoV-2, Journal of Virological Methods, 2023


AIM
During the course of the COVID-19 pandemic, nasopharyngeal swabs, combined throat and nose swabs and saliva samples have been evaluated for SARS-CoV-2 detection using nucleic acid amplification tests (NAT). Literature on anterior nasal swabs is limited and comparative studies often group anterior nasal and mid-turbinate swabs together. This study investigated a novel anterior nasal swab, Rhinoswab™, that has been designed to standardize self-collection, maximise sample uptake and improve user comfort.
METHOD
Twenty two symptomatic adult participants were recruited at St Vincent’s Hospital Melbourne and tested on days 2, 4 and 11. The study used combined throat and nose swabs and neat saliva samples as the comparators. SARS-CoV-2 detection was performed using the AusDiagnostics SARSCoV-2/Influenza/RSV 8 well assay.
RESULTS
In the study Rhinoswab™ performed equally well in comparison to a combined throat and nose swab for the laboratory detection of SARS-CoV-2 using nucleic acid amplification techniques.
Financial sponsor: Rhinomed
Rhinoswab’s viral load transfer comparable to traditional swabs1
An independent laboratory, Gnomix (Adelaide, Australia) was engaged to compare the elution efficiency of the Rhinoswab™ compared to a traditional nasal swab (Copan ESwab™).
The study found that the CT scores for the two swabs were comparable at 20 μl loading for both high and low virus burdens.
| Rhinoswab | ESwab™ | |
| High Virus Burden 20 μl (1ml Elution) Average Ct | 25.45 (± 0.24) | 25.75 (± 0.43) |
| Low Virus Burden 20 μl (1ml Elution) Average Ct | 29.15 (± 0.24) | 30.39 (± 1.03) |
The study also evaluated the sample yield or average sample recovery for the Rhinoswab compared to a traditional nasal swab (Copan ESwab™).
| Rhinoswab | ESwab™ | |
| High Virus Burden 20 μl (1ml Elution) | 16.34 μl (82%) | 14.50 μl (73%) |
| Low Virus Burden 20 μl (1ml Elution) | 21.80 μl (~100%) | 17.33μl (87%) |
These results suggest a superior elution efficiency for the Rhinoswab when comparing identical initial loadings of both the high and low virus burden sample.
SARS-CoV-2 detected with 100% accuracy when rt-PCR test applied to infected Rhinoswab samples1
A SARS-CoV-2 spiked study was conducted at the Victorian Infectious Diseases Reference Laboratory (VIDRL) at the Peter Doherty Institute for Infection and Immunity.
The study evaluated the efficacy of Rhinoswab in transferring a viral load for testing compared to a commercially available standard flocked swab (Copan ESwab™) using reverse transcription-polymerase chain reaction (rt-PCR).
Both Rhinoswab and the Copan ESwab™ reported 100% accurate diagnosis of SARS-CoV-2
Expert opinion
LEADING researchers AGREE ANTERIOR OR MID-TURBINATE (DEEP NASAL) SWAB IS AN ACCEPTABLE SPECIMEN COLLECTION METHOD FOR respiratory viruses
According to several recent peer-reviewed studies and scientific papers that have compared nasal swabs to nasopharyngeal (NP) or Oropharyngeal (OP), self-collected mid turbinate nasal swabs (supervised or at-home) represent a reliable alternative to NP and OP swabs collected by healthcare workers for the detection of SARS-CoV-2, flu and other common respiratory viruses.
Based on evidence such as this, both the US CDC and the FDA have approved self-collected nasal sampling (supervised or at-home) as an acceptable specimen collection method for SARS-CoV-2 testing and other respiratory viruses.
Usability
Usability study results
Self collection with rhinoswab is easy and provides consistently reliable samples for testing
In a usability study conducted by Rhinomed over 90% of users found the device to be easy or very easy to use to use as part of a self-collection kit for viral testing.1
Rhinoswab’s novel design standardizes its placement within the nose, facilitating the collection of a quality sample, no matter the user group1 (n=32).
Rhinoswab is suitable for use across a range of clinical and non-clinical settings
Collection by a healthcare worker or supervised self-collection in a clinical setting
- COVID-19 testing clinics
- Hospitals
- GP clinics
- Pathology centres
Self collection in a non-clinical setting (where local guidelines permit)
- At work, especially in high risk occupations requiring high frequency testing
- Factories
- Abbatoirs
- Airlines
- At place of study
- Universities/colleges
- Schools
- At home
Rhinoswab is a safe alternative to traditional swabs1
- There have been no adverse outcomes associated with Rhinoswab to date, other than occasional light nasal spotting when the swab is removed1
- There are no specific contraindications for collecting samples with Rhinoswab. However, similar to all other swabs11, clinicians should be cautious if the patient has had recent nasal trauma or surgery, has a markedly deviated nasal septum, has a history of chronically blocked nasal passages or severe coagulopathy, or is taking anticoagulant therapy
- Rhinoswab’s positioning in the low-mid nasal turbinates reduces the likelihood of sneezing during sample collection and avoids the risk of coughing/gagging that comes with NP/OP swabs. This mitigates the risk of aerosol generation at time of sampling, decreasing the infection risk to any healthcare workers in attendance3,12,13
User Experience
Studies show superior comfort & ease of use drive strong preference for rhinoswab
Netherlands User Testing, 2021
A user experience study was conducted in 2021 by the Canisius Wilhelmina Hospital (CWZ) and the Radboud University Medical Center (Radboudumc) in the Netherlands, with the support of the Municipal Health Authority (GGD Gelderland Zuid) at their COVID-19 mass testing sites.
People were invited to take part in the study after a nasopharyngeal swab sample had been taken by a healthcare worker for covid testing. In total, 556 people agreed to self-test with Rhinoswab and of these 302 participants completed an online survey providing comparing and providing feedback on their experience with Rhinoswab and the traditional sampling method.
A summary of the study results are shown below:
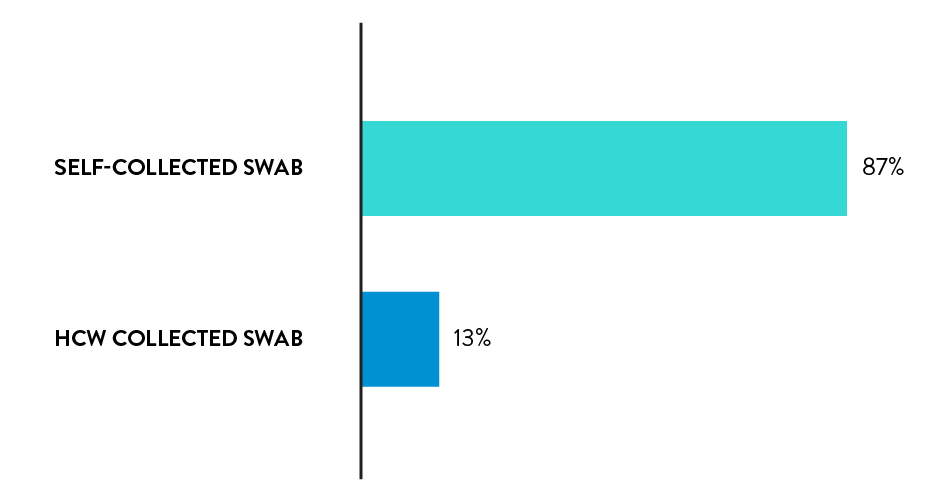
Rhinoswab is the preferred sample collection method
87% of participants chose Rhinoswab as their preferred sample collection method for future covid tests.
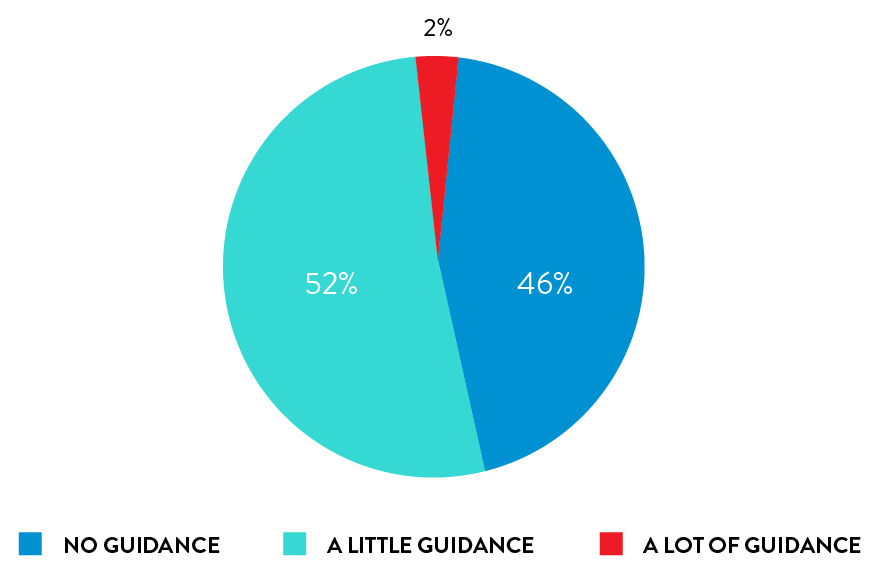
Self-collection with rhinoswab requires little or no guidance from HCWs
98% of participants required little or no guidance when from a healthcare worker when using Rhinoswab.
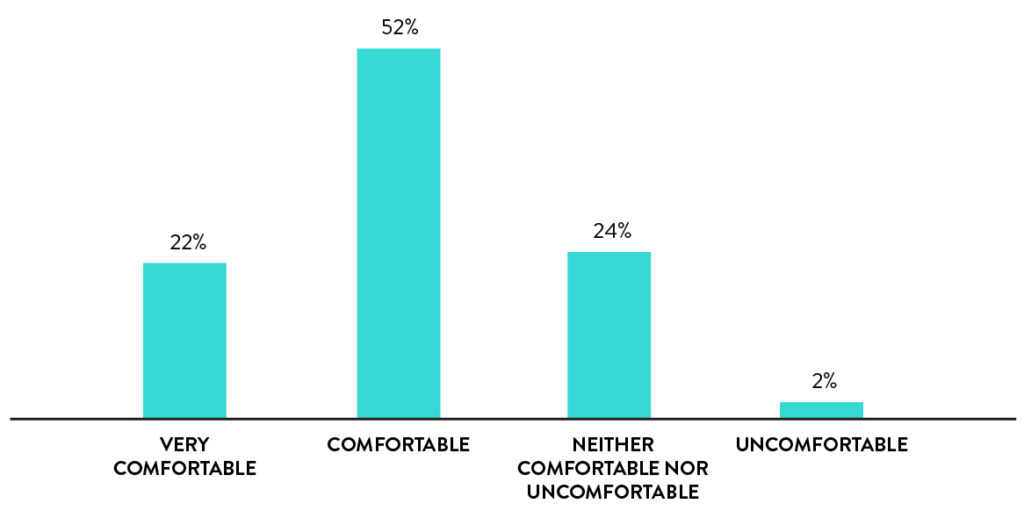
Self-collection with rhinoswab is comfortable
74% of participants found Rhinoswab to be comfortable or very comfortable and only 2% rated it as uncomfortable.
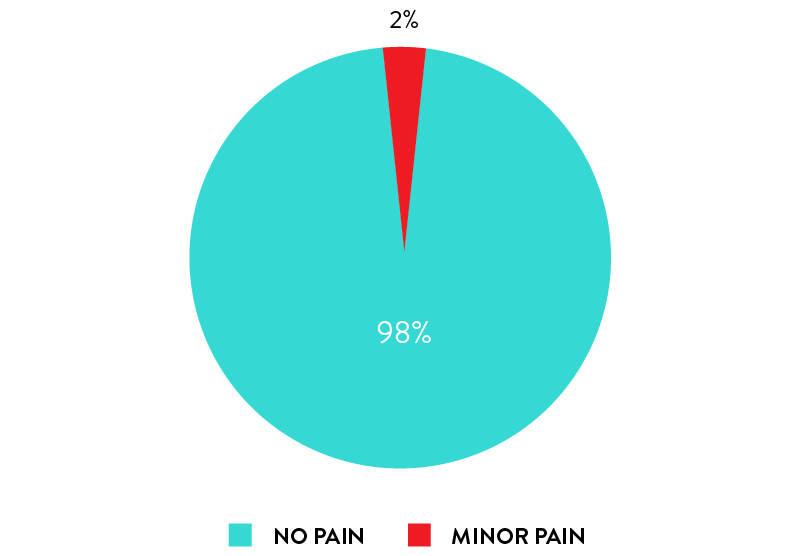
Self-collection with rhinoswab is pain free
98% of participants reported they experienced NO DISCOMFORT or PAIN when using Rhinoswab
RHINOSWAB’S SUPERIOR COMFORT MAY HELP REDUCE TESTING FEARS, OVERCOMING RELUCTANCE TO GET TESTED
Anecdotal reports indicate that some individuals avoid or delay testing due to the pain/discomfort that may be experienced with traditional swabs.10
The superior comfort of Rhinoswab has the potential to improve patients’ willingness to undergo testing, thereby enhancing viral testing rates.
Financial sponsor: Rhinomed
NSW Health Pathology Testing, 2021
NSW Health Pathology tested 19,000 Year 12 students as they attended Qudos Bank Arena to receive their covid vaccinations in August 2021.
Students self collected a nasal swab using Rhinoswab and the test was conducted using rapid pcr.
Students were also invited to take part in an online survey about the sample collection process. The aim of the study was to explore the Rhinoswab user experience and compare that with health care collected sampling methods they may have experienced in the past.
2532 students completed the survey, with 1190 having had health care collected swabs in the past.
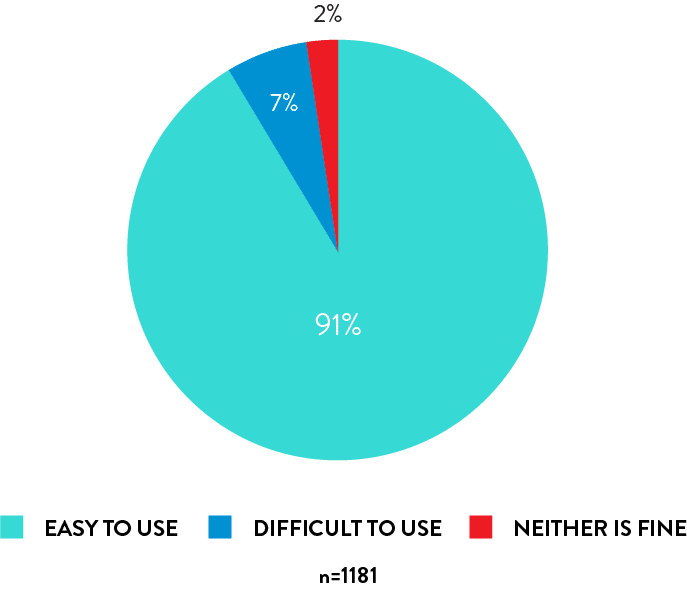
Rhinoswab is easy to use
91% of students found self collection with Rhinoswab easy to use.
Rhinoswab was preferred
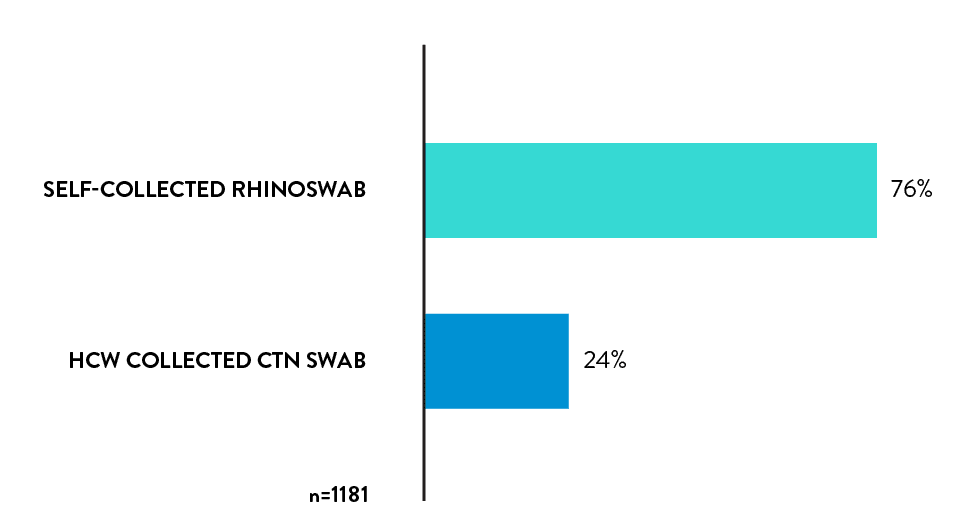
76% of the respondents who have previously had HCW collected swab, would prefer Rhinoswab for future tests.
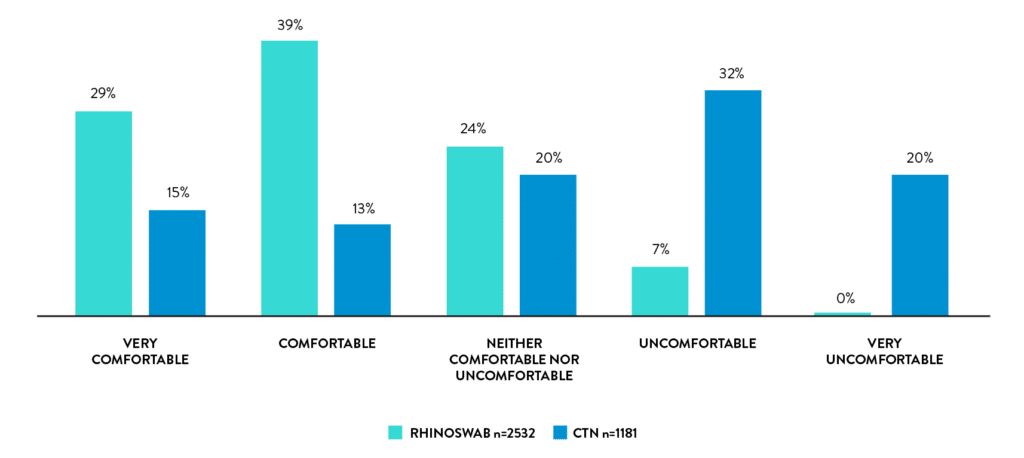
Self-collection with rhinoswab is comfortable
68% of students found Rhinoswab comfortable versus 28% for HCW collected swabs.
Lab considerations
Rhinoswab fits with existing pathology workflows and standard rt-PCR collection vials
- Rhinoswab does not require any change to your pathology workflows and can be immediately integrated with your existing transport media and collection tubes
- Rhinoswab can be included in your self-collection kits, with easy-to-follow written and video instructions
- Rhinoswab samples are also suitable for many rapid antigen tests that can accommodate Rhinoswab’s unique design
Reliable supplier, comparable pricing1
- Medical grade quality; polycarbonate handle with flocked nylon tips
- Comparable price to traditional swabs
- FDA registered (Class I)
- Suitable for individuals 16 years or older; paediatric size available in near future
- Available in shipper carton with 2 bags each containing 500 swabs (total 1000 swabs)
- Further resources such as technical specifications and datasheets to support the procurement process within your hospital, health service or organization are available upon request or can be downloaded here.
FAQs
Yes. Rhinoswab and Rhinsowab Junior can be used with rt-PCR testing.
Rhinoswab is compatible with most existing pathology workflows and standard transport media and collection tubes. In some cases, automated liquid handling equipment require a change of script for the adult swab.
Yes. Rhinoswab is listed for sale as a Class 1 device in Europe (CE Mark), in the United States with the US Food and Drug Administration (FDA), in the UK with the Medicines and Healthcare products Regulatory Agency (MHRA), in Canada with a Medical Device Establishment Licence (MDEL) and in Australia with the Therapeutic Goods Administration (TGA).
Rhinoswab may be administered directly by a healthcare professional, or self-administered by adolescents (12 years and older) and adults.
Users under the age of 16 years, or those challenged by vision, cognitive, dexterity, literacy or language limitations, may require the assistance of an adult aged 21 years and over.
Rhinoswab Junior is designed for children aged 4 years and over.
Both Rhinoswab and Rhinoswab Junior have been validated in clinical trials as comparable to the standard combined throat and nose swab.
No. Rhinoswab and Rhinoswab Junior are self administered.
Rhinoswab is designed to be self administered to minimise risk to health care workers. Health care workers may supervise the sample collection process if necessary for those who are under 16 years of age or those who have cognitive or other challenges.
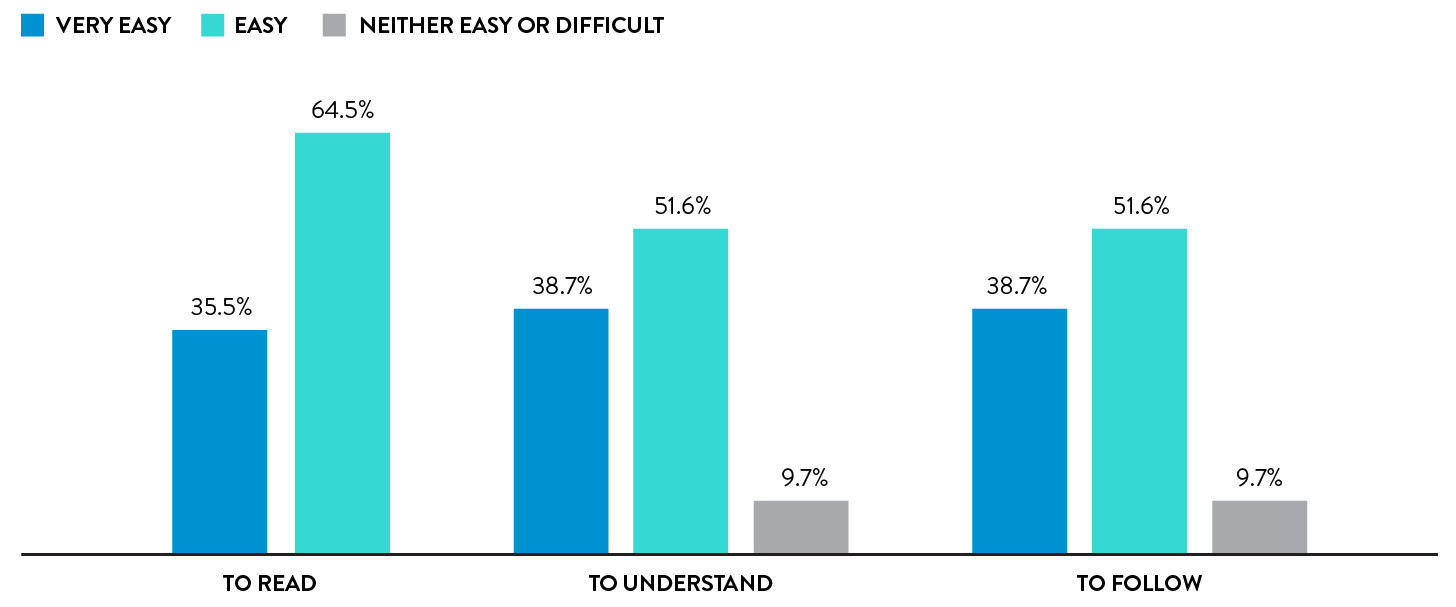
Yes. A usability study showed that 77.1% of participants found the swab to be very comfortable or comfortable and a further 20.8% found it to be neither comfortable nor uncomfortable.
In a study of 53 children, 89.8% described Rhinsowab Junior as comfortable or a little uncomfortable, and 88% prefer Rhinoswab Junior over combined nose and throat swabs for future tests.
No. A usability study showed that 97.9% of participants (47 out of 48 participants who took part in the trial) reported that they experienced no pain at all when using the swab. Further, a user experience study conducted in 2021 by the Canisius Wilhelmina Hospital (CWZ) and the Radboud University Medical Center (Radboudumc) in the Netherlands, found that 98% of participants reported they experienced NO DISCOMFORT or PAIN when using Rhinoswab.
In a study of 53 children, 89.8% described Rhinsowab Junior as comfortable or a little uncomfortable, and 88% prefer Rhinoswab Junior over combined nose and throat swabs for future tests.
Yes, nasal swabbing is a valid sample collection method.
Swabbing only the nose is a valid sample collection approach. Scientific literature and guidance provided by the FDA, CDC (Center for Disease Control) and WHO support the efficacy and reliability of nasal swabs that access just the front of the nose rather than the depth of the nasal cavity.
No. Rhinoswab is not a test.
There are three stages to conducting a covid test; 1) Collect a sample 2) analyse the sample 3) report the results. Rhinoswab provides a comfortable alternative to standard nasopharyngeal swabs for collecting the sample. Once Rhinoswab collects a sample from the nose, the swab is placed in a standard vial containing saline transport media and sent to a lab for testing. The test is conducted within the pathology laboratory using rt-PCR testing equipment.
The Office of the Assistant Secretary of Health SARS – Co-V2 (COVID-19) Fact Sheet states: “Collection of nasal swab specimens is less technically complex, so can reduce the risk of the spread of infection to healthcare providers, by (1) reducing the duration of the procedure, and (2) allowing the patient to perform self-collection while under supervision.”
Self collection of nasal swab samples are safer for Health Care Workers who can supervise patients performing self collection from a distance rather than the HCW performing the collection.
The Office of the Assistant Secretary of Health SARS – Co-V2 (COVID-19) Fact Sheet recognizes that self collection of nasal samples “… also lessens PPE utilization, given that the patient can perform self-collection under supervision (versus the health care provider performing the collection).”
If you would like to trial Rhinoswab to determine how well it performs on your equipment or in your laboratory, please contact Peter Jordan, SVP Business Development, at pjordan@rhinomed.global to arrange a trial.
Downloads & Specifications
PDF DOWNLOADS
Rhinoswab Instructions for Use (IFU)
Rhinoswab Safety Data Sheet
Rhinoswab NEWS
- European Launch of Revolutionary New Swab for Testing Children for Respiratory DiseasesThe world’s most child-friendly respiratory swab was launched in Europe today at Medica 2022 in Dusseldorf, Germany. Rhinoswab Junior is the first nasal swab developed specifically for children. Its unique design addresses one of the pandemics’ … Read more
- New study says there is a better swab for testing children for covid and other virusesA new study suggests the traditional swab suffers from strong reluctance for use for children and a new Melbourne-designed Rhinoswab Junior is the preferred method of sample collection. The reluctance to test children for COVID-19 … Read more
- Study confirms new nasal swab is most accurate, comfortable and easy for kidsRhinomed is pleased to advise of the forthcoming publication of a further study and data by researchers from the Murdoch Children’s Research Institute and the Royal Children’s Hospital (RCH) Melbourne confirming the efficacy and comfort of the … Read more
- Surescreen Australia & Rhinomed – Rhinoswab Supply DealRhinomed Limited is pleased to report that it has finalised an exclusive supply agreement with SureScreen Australia to supply Rhinoswabs and Rhinoswab Juniors for inclusion in SureScreen’s class-leading range of lateral flow point of care … Read more
REquest a sample
Rhinoswab by Rhinomed is FDA, TGA and CE registered and available for trial or purchase. Rhinoswab is currently available for professional and commercial use only and comes in a minimum order quantity of 1000 swabs.
If you would like to trial Rhinoswab, please complete this form to request samples.
Or contact us to discuss how Rhinoswab may help deliver a better sampling solution for your needs.

References
- Rhinomed Pty Ltd. Data on file 2020/21.
- Tu YP et al. Swabs collected by patients or health care workers for SARS-CoV-2 testing [Letter to the Editor]. N Engl J Med; 3 June 2020.
- McCulloch D. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Network Open 2020;3(7):e2016382. doi:10.1001/jamanetworkopen.2020.16382.
- Wehrhahn M et al. Self collection: an appropriate alternative during the SARS-CoV-2 pandemic. J Clin Virol 2020;128:104417. doi: https://doi. org/10.1016/j.jcv.2020.104417.
- Altamirano J et al. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for severe acute respiratory syndrome coronavirus 2 testing. JAMA Netw. Open 2020;3(6):e2012005. doi:10.1001/jamanetworkopen.2020.12005.
- Centers for Disease Control and Prevention (CDC), US Department of Health & Human Services. Interim guidelines for collecting, handling and testing clinical specimens for COVID-19 (last updated 6 Jan 2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html#specimen (last accessed February 2021).
- US Food and Drug Administration (FDA). Coronavirus (COVID-19) update: daily roundup (published March 23, 2020). Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup (last accessed February 2021).
- Office of the Assistant Secretary for Health, US Public Health Service. Nasal (Anterior nasal) specimen collection for SARS-CoV-2 diagnostic testing. Fact sheet. 11 Nov 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/lab/OASH-nasal-specimen-collection-fact- sheet_updates_2020_11_11_508.pdf (last accessed February 2021).
- Chicago Sun Times, 10 April 2020. COVID-19 tests: What it feels like to have one. Available at https://chicago.suntimes.com/coronavirus/2020/4/10/21216845/coronavirus-covid19-abbott-rapid-swab-test-nose-nasal-how-it- feels-jayant-pinto-molly-erickson (last accessed February 2021).
- ABC News, 27 August 2020. Coronavirus testing in Melbourne went down — here’s why some Aussies are avoiding the swab. Available at https://www.abc.net.au/news/2020-08-27/coronavirus-covid-19-test-victoria-queensland- nsw-why-not/12601334?nw=0 (last accessed February 2021).
- Marty FM, Chen K and Verrill KA. How to obtain a nasopharyngeal swab specimen. N Engl J Med 2020;382:e76. doi: 10.1056/NEJMvcm2010260.
- Palmas G et al. Nasal swab as preferred clinical specimen for COVID-19 testing in children. Pediatr Infect Dis J 2020;39(9):e267−270. doi: 10.1097/INF.0000000000002812.
- Harding H et al. Aerosol-generating procedures and infective risk to healthcare workers from SARS-CoV-2: the limits of the evidence. J Hosp Infect 2020;105(4):717–725. doi:10.1016/j.jhin.2020.05.037
- Arnaout R et al. SARS-CoV2 testing: the limit of detection matters. bioRxiv (preprint); 4 June 2020. doi: 10.1101/2020.06.02.131144.
- Dhiman N et al. Effectiveness of patient-collected swabs for influenza testing. Mayo Clin Proc 2012;87(6):548–554. doi:10.1016/j.mayocp.2012.02.011.