An independent laboratory, Gnomix (Adelaide, Australia) was engaged to compare the elution efficiency of the Rhinoswab compared to the standard of care nasal swab (Copan ESwab™).
Method
An aliquot of gamma-irradiated (inactivated) SARS-CoV-2 virus with a nominal CT value of 18 (assay dependent) was diluted 1/200 in a stock solution of donated saliva to represent a high virus burden sample and 1/2000 in to represent a low virus burden sample.
An aliquot of gamma-irradiated (inactivated) SARS-CoV-2 virus with a nominal CT value of 18 (assay dependent) was diluted 1/200 in a stock solution of donated saliva to represent a high virus burden sample and 1/2000 in to represent a low virus burden sample.
Two protocols were followed:
- To reflect the standard of care, the high and low virus burden samples were applied as 4 x 5μl spots (20μl) onto 10 Rhinoswabs and 5 standard of care nasal swab (Copan ESwab™).
- To evaluate the inherently greater potential capture area of the Rhinoswab (with dual swab heads) in comparison to the standard of care nasal swab (Copan ESwab™) 4 x 8μl spots (32μl) were applied onto 10 Rhinoswabs.
In both instances each swab was then placed into a 5ml tube containing 1ml of Saline, vortexed vigorously for 30 seconds and left to elute for 1 hour at room temperature.
Following elution, 140μl of eluate was extracted and reverse transcription and qPCR were performed using the QuantiNova Pathogen +IC Kit (QIAGEN) in combination with the SARS-CoV-2 N1+N2 assay kit (QIAGEN). The QuantiNova IC RNA, extraction negative control and PCR negative control were included on each run.
RESULTS
PROTOCOL 1 – AT STANDARD LOAD
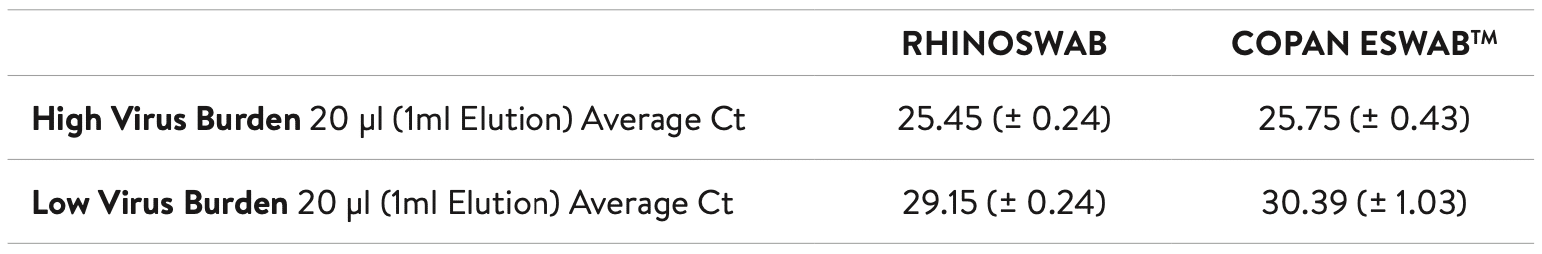
The study found that the CT scores for the two swabs were comparable at 20 μl loading for both high and low virus burdens.
The study also evaluated the sample yield or average sample recovery for the Rhinoswab compared to the standard of care nasal swab (Copan E-Swab™).

These results suggest a superior elution efficiency for the Rhinoswab when comparing identical initial loadings of both the high and low virus burden sample.
PROTOCOL 2 – At Greater Load Potential

These results suggest that it is possible to recover more virus from the extra loading capacity, at both high and low virus burden, although there appears to be slightly diminished overall efficiency.
Discussion
Under the conditions tested and with the materials supplied, the Rhinoswab demonstrated not only a comparable but also a superior elution efficiency to the commercially available Standard of Care nasal swab (Copan ESwab™) at both 20μl at High Virus burden and 20μl at Low Virus burden.
The study also showed it was possible to recover more virus from the extra loading capacity of Rhinoswab.